
The 2024 FESPA Party Night on Thursday 21 March 2024
The FESPA Party Night is the ultimate event for the industry to network, unwind and enjoy an evening of live entertainment, music, drinks and grea [...]

Avoid panic buying of cotton, says SIMA
Spiraling of cotton prices – avoid panic buying – SIMA
Coimbatore: The predominantly cotton based Indian textiles & clothing industry has been [...]

Industry Associations Joint Letter On The EU-Mercosur Agreement
Industry Associations' Joint Letter seeking rapid conclusion of the EU-Mercosur Agreement
Brussels – EURATEX and 22 industry associations sent a joi [...]

European Technical Textile Industry faces Tough Times
A diverse group of workers operating high-tech machinery in a textile factory, with raw materials being transformed into finished products, symboliz [...]

International Conference on Sustainability and Circularity
International Conference On Sustainability & Circularity: The New Challenges For The Textile Value Chain
Wednesday, 31st January 2024
Hotel The [...]

EURATEX Manifesto for Making the Industry Competitive
2024 is a Turning Point for the European Textiles and Clothing Industry
From 6 to 9 June 2024, European citizens will vote for a new European P [...]

Prolonged recession in the global textile value chain
Weakening demand, inflation, and geopolitics – major causes for prolonged recession in the global textile value chain – ITMF Chief
The textile [...]
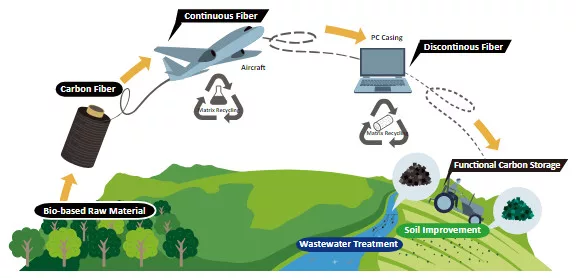
Toray Carbon Fiber Recycled from Boeing 787 Wing Production
Toray Carbon Fiber Recycled from Boeing 787 Wing Production Process Applied in Lenovo ThinkPad X1 Carbon Gen 12
Tokyo, Japan – Toray Industries, In [...]

Thermal fabric cutting system
Thermal fabric cutting system - Weftmaster Loepfe's CUT-iT for airbag fabrics
WeftMaster CUT-iT for airbag fabrics. In case of the unexpected whi [...]

Hohenstein and Under Armour present test kit for textile microfibre shedding
For the test, the textiles are cut into round pieces and stirred by magnetic mixers in a glass of liquid. After this process, the fibers are filte [...]