Indigo is a so-called vat dye, which means that it needs to be reduced to its water soluble leuco-form before dyeing. The reduced form is absorbed into the fibres, and when oxidized back to its blue form it stays within the fibre. Earlier the reduction and dyeing was done with fermentation. Nowadays, the most of the reduction has been done chemically by sodium dithionite. It is considered environmentally unfavourable since it produces sulphite, sulphate, thiosulphate and toxic sulphides as degradation products, which then contaminate the waste waters from the dyeing plants. Therefore there has been interest to find new possibilities to reduce indigo.
Indigo is a so-called vat dye, which means that it needs to be reduced to its water soluble leuco-form before dyeing. The reduced form is absorbed into the fibres, and when oxidized back to its blue form it stays within the fibre. Earlier the reduction and dyeing was done with fermentation. Nowadays, the most of the reduction has been done chemically by sodium dithionite. It is considered environmentally unfavourable since it produces sulphite, sulphate, thiosulphate and toxic sulphides as degradation products, which then contaminate the waste waters from the dyeing plants. Therefore there has been interest to find new possibilities to reduce indigo.
Indigo has been used in dyeing textile materials for thousands of years. Because indigo pigment is insoluble in water, it must be de-oxidized, or “reduced,” to a water-soluble white form known as “leuco indigo” before it can be used in dyeing. Leuco indigo is unstable; it oxidizes and returns to its blue pigment form when exposed to oxygen. Thus, leuco indigo solution needs to be kept in an oxygen-free environment, or otherwise stabilized, if it is not being used immediately for dyeing.
For many years, dyehouses commonly reduced indigo in-house through a process known as hydrosulfite reduction. Dyers created a “stock vat,” in which indigo is reduced in water with sodium hydrosulfite and solubilized with an alkali, e.g., sodium hydroxide. The resulting leuco indigo solution is then transferred into a feed tank and fed into the dyebath. After the dyebath is prepared, the textile material is dyed through a process known as “dipping” and “skying.” In “dipping,” the textile material is contacted with leuco indigo in the dyebath; in “skying,” the dyed textile material is introduced to the air, causing the indigo to convert back to its blue pigment form.
A second common method of indigo reduction, catalytic hydrogenation, was patented by Andre Brochet in 1917. See U.S. Patent No. 1,247,927 (“Brochet”). The superficial difference between hydrosulfite reduction and catalytic hydrogenation is that the latter uses gaseous hydrogen, rather than sodium hydrosulfite, as a reducing agent. Catalytic hydrogenation allowed “economical production of concentrated solutions of leuco derivatives free from impurities and mineral salts”; when left to settle, the solution naturally separates from nickel or another catalytic metal and can be “drawn off and is ready for use”.
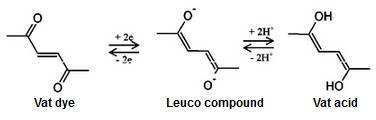
As a vat dye indigo needs to be reduced to its water-soluble form before it can be used in dyeing. The reduced form is called the leuco compound, leuco coming from the Greek word leucos meaning white, which refers to the change of colour of the vatting liquid after reduction. With indigo the colour of the leuco-indigo solution is yellow green when reduced with sodium dithionite. Several different methods have been invented for the indigo reduction and dyeing, all starting from the fermentation vat, which was used for centuries before the modern technology came. The fermentation process was done in large wooden barrels called vats from which the name for the vat dyes also comes. There were also urine vats which used stale urine as an ingredient in the fermentation. The industrial revolution brought new possibilities to the dyeing with such methods as “copperas” method which combined ferrous sulphate with slaked lime or potash and zinc-lime vat in which slaked lime and zinc powder interacted to form hydrogen as a reducing agent. The universal reducing agent sodium dithionite was finally introduced to the vat dyeing at the end of the 19th century.
Most important to the dyehouses, however, was the fact that Brochet’s leuco indigo solution could be stabilized in solid form, usually powder or paste, and coated with molasses or glue to protect the reduced indigo from air and prevent premature oxidation. This allowed the indigo reduction process to shift out of the dyehouses and into chemical manufacturers, which began to produce and sell prereduced indigo to dyehouses in the early 1900s. Rather than create a stock vat, dyers needed only to dissolve the prereduced indigo into a preparation tank, add caustic soda (i.e., sodium hydroxide) and sodium hydrosulfite to remove oxygen from the water, and transfer the resulting solution from the preparation tank to the dyebath. This significantly reduced the time necessary to prepare a dyebath, the dyehouses’ expenditures on sodium hydrosulfite & caustic soda, and the level of pollution in dyehouse waste water and on shopfloors.
Dyeing with pre-reduced Indigo
This specially formulated indigo is already 60% reduced, therefore, it allows you to use Soda Ash instead of nasty Lye in the dye vat. Extremely easy to use, Pre-Reduced Indigo makes setting up an Indigo vat almost effortless. There is no need to grind, then paste up the Indigo granules because they dissolve easily in water. The vat can be prepared in about 10 minutes!

This specially formulated indigo is already 60% reduced, therefore, it allows you to use soda ash instead of lye in the dye vat. Extremely easy to use, pre-reduced indigo makes setting up an indigo vat almost effortless. There is no need to paste up the indigo granules because they dissolve easily in water. When indigo is pre-reduced, the dye substance has already been combined with reduction chemicals and dried. It simply needs to be added to water with small amounts of chemicals to maintain reduction. Dyeing takes place in just a few minutes, and color is excellent without the need for multiple immersions.
Precautions : Please use an organic fume respirator when working with the Thiourea Dioxide (AKA Dyehouse Color Remover aka Thiox) or Sodium Hydrosulfite. Do not use utensils or dishes used for eating. Wear gloves. Always work in a well ventilated area.
When using bulk pre-reduced Indigo, the following indicative recipe may be used :
Pre-Reduced Indigo : 1.0 Kg
Thiourea Dioxide or Sodium Hydrosulfite : 3.2 Kg
Soda Ash : 4.8 Kg
Water : 180 Litres