Preparation of Cotton Fiber Based Adsorbent and Its Adsorption Performance for Metal Ions
Country: China
Abstract:
In this paper, a new type of cotton fiber based adsorbent is prepared by selectively oxidizing cotton fiber, and then grafting the HBP—OH and β-cyclodextrin. Studies on the adsorption of heavy metal ions (Pb2+, Cd2+, Cu2+) showed that the prepared cotton fiber based adsorbent had a higher adsorption capacity than the raw cotton fiber. Its adsorption capacity is 12-20 times that of raw cotton fiber.
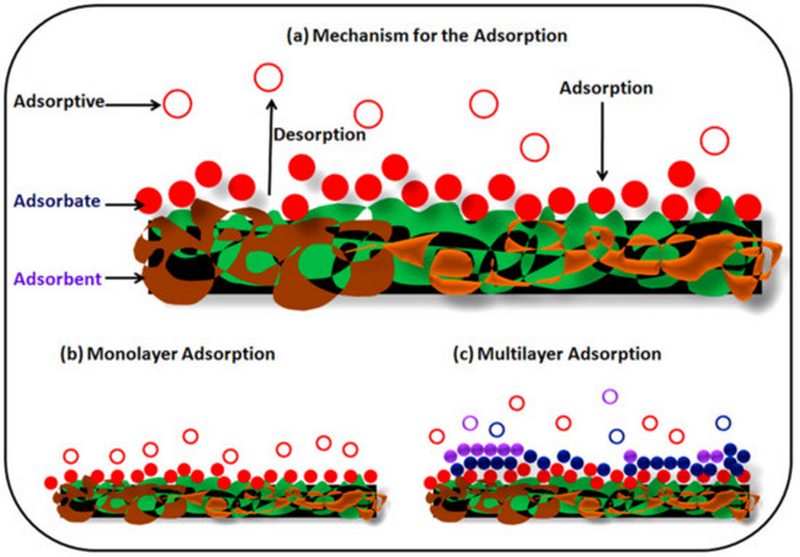
The adsorption process is the most efficient and admirable process for the treatment of toxic metals present in wastewater. In this process, toxic material is shifted by physical or chemical means onto the available surface of the adsorbent. The adsorption process is a cheap method and has a very low operating cost, and causes less contamination during the extraction process of toxic metal in comparison to conventional methods. In the adsorption methods, sorbents can be regenerated as well as reused several times for effective removal, and hence are considered to be an environmentally friendly method. The major characteristics required for the choice of adsorbents are price effectiveness, large surface area, pore size distribution, presence of functional moiety, and polar characteristics of the sorbent which determines the efficiency of adsorption methods. It is, therefore essential to understand the adsorption process. Adsorption is a mass transport method of solute present in the solution and accumulated onto the surface of the adsorbent which is generally a solid substance. There are two kinds of force i.e., physical and chemical interactions that exist between adsorbent and adsorbate. Physical forces are weak and adsorbed molecules can be attached to adsorbents anywhere, meaning physical forces are non-specific in nature. Chemical adsorption is specific in nature and adsorbate binds to adsorbents through the covalent or electrostatic bonds. In the case of physical adsorption, forces are Van der Waals, dispersion interactions, and hydrogen bonding.
 (a) General mechanism for the adsorption, (b) monolayer adsorption, and (c) multilayer adsorption. |
|